What are the advantages of living in a biofilm?
A Bacterium’s Eye View
The nearly universal existence of biofilms suggests that living in this "lifestyle" carries some pretty significant payoffs. As far as we can tell, biofilms have been and remain the dominant life forms on the planet. To have persisted in such abundance over such a lengthy period of time implies that this is a very advantageous way of life. Although not exhaustive, the following may represent some of the major benefits associated with biofilm formation.
Nutrient Scavenging
Many, perhaps most, biofilms grow in low nutrient or oligotrophic conditions. The EPS of the biofilm matrix is negatively charged and hydrophobic. These properties enable the biofilm to concentrate ions and dissolved organic carbon compounds from the oligotrophic bulk fluid. Thus biofilms can grow in nutrient conditions that do not permit the growth of planktonic cells. This phenomenon was first observed by ZoBel (1943) in his classic bottle experiments. Bacteria were added to a bottle containing a nutrient concentration too limited to support planktonic growth. The number of bacteria in the fluid portion of the system decreased and it was found that they had attached themselves to the wall of the container. Vigorous agitation put many of the cells back into the fluid phase and it was observed that their numbers had actually increased. The bottle's surface and perhaps the developing biofilm matrix caused nutrients to adsorb to the glass in sufficient concentrations to permit reproduction.
A Broader Range of Habitats Available for Colonization
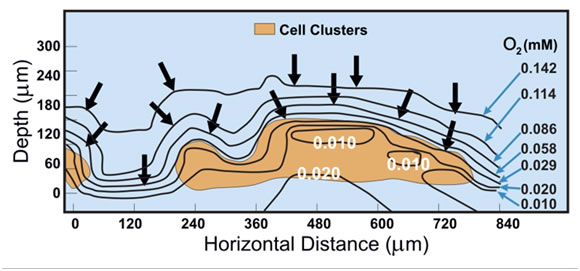
In the laboratory, biofilms are often, although not always, grown in monoculture. Even in these single species biofilms there have been shown to be a great range of microhabitat niches. The use of techniques such as confocal microscopy in combination with physiologically responsive fluorescent dyes, and microelectrodes which can be placed at various positions and depths with in a biofilms, has shown that there are steep gradients of many growth factors, including oxygen and sulfide, and hydrogen ion concentration (pH).
As shown in Figure 1, this x-z representation and using microelectrode data, the concentration of O2 in the bulk fluid and at the surface of the biofilm is 14 (mM) , while that in the center of the biofilm mass is essentially anaerobic. In moving from the bulk fluid to the interior of the biofilm oxygen levels drop rapidly producing zones of oxygen concentration from aerobic to strongly anaerobic. These differences are largely due to diffusion restriction and metabolic consumption by aerobic and facultative bacteria within the biofilm.
Fluorescent molecular probes can be combined with confocal microscopy to reveal the physiological condition of bacteria with in the biofilm. Redox sensitive probes such as fluorescine can indicate the level of metabolic activity within individual cells or masses of cells. Other reagents, such as the Live/Dead® stain produced by Molecular Probes, will differentially stain cells depending on the integrity of their cell membrane. Living cells are seen as green while dead ones stain red.
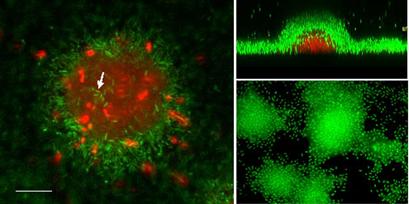
Cells in the center of micro-colonies undergo complex differentiation; subpopulations of cells die and undergo cell lysis as indicated by red-stained dead cells. Other cells inside micro-colonies survive the killing process (arrow, green cells), and are often observed to disperse from the interior of the micro-colony. Bar = approx. 50 µm.
Bacteria living at the surface of a biofilm or near a fluid conducting channel will experience a very different set of environmental conditions than those growing even a short distance away within the biofilm mass. The situation is complicated by the fact that microbial waste products may accumulate within the depths of the biofilm but may be carried away by bulk fluid flow at or near the surface. Add a second or third microbial component to the biofilm community and the situation becomes much more complex. Typically, minority species within the biofilm are not uniformly distributed but grow in specific favorable locations with in the mass. With two or more species the various gradients caused by nutrient utilization and waste metabolite excretion will produce a community with an astonishing array of microhabitats in which specific microorganisms may find suitable growth conditions.
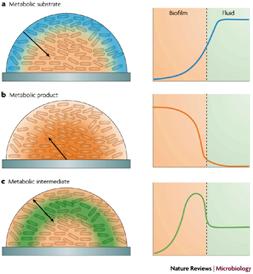
Three qualitatively distinct patterns of chemical heterogeneity arise in biofilms owing to reaction-diffusion interactions for a metabolic substrate (blue; a), a metabolic product (orange; b) and a metabolic intermediate (green; c). The concentration of a substrate that is consumed in the biofilm decreases with depth into the biofilm and distance away from the source (a). Conversely, a metabolic product is more concentrated inside the biofilm (b). A metabolic intermediate that is both consumed and produced within the biofilm can exhibit concentration profiles that have local maxima (c). Consider the facts that 1) several biofilm components may be in competition for a given metabolic substrate, that 2) the waste product of one species may be the metabolic substrate of another, that 3) waste products of a given species A may be inhibitory unless removed by diffusion or metabolism by a second species B and that 4) metabolic intermediates may be used in various ways by different species and one begins to understand that even a biofilm with only a few component species may represent a very large diversity of microhabitats.

