A substantially updated version of the hypertextbook is available here. Please migrate to that version. This one will eventually disappear.
Quorum Sensing and Cell/Cell Signaling in Bacteria
Preface
This page describes the phenomenon of autoinduction and cell/cell signaling first described some thirty years ago and now recognized as a major element in the ability of bacteria to respond to changing conditions in their ecosystem. The nature of quorum sensing, the genetics, molecular biology and ecology of the some of the best studied systems are discussed. In addition, three exercises are described which can be used to demonstrate the nature and ubiquitousness of quorum sensing phenomena. The first describes a system in which naturally occurring autoinducing compounds can be detected and the second outlines a cell density dependent system using bioluminescent organisms in which the basic principles and specificity of quorum sensing signaling can be demonstrated.
Introduction
The dynamic nature of the bacterial phenotype depends largely on selective gene expression. The expression of an advantageous phenotype must therefore allow survival and proliferation within the constraints of the growth environment. Such evolutionary pressure has resulted in a network of sensor mechanisms that transduce environmental stimuli into gene expression, and hence a phenotype complementary to the prevailing environmental conditions.
In retrospect it seems surprising that one of the most remarkable discoveries in the history of microorganisms began with a fish.
In the 1970s, scientists at Harvard University and Scripps Institute of Oceanography began to report on a unique and fascinating phenomenon. The system they were investigating was the production of bioluminescence by the marine bacterium Vibrio fischeri (then called Photobacterium). This bacterium which lives free in the ocean also lives in large numbers in the light emitting organs of the flashlight or lantern fish (Photoblepharon palpebratus). Living planktonically in the ocean Vibro fischeri does not bioluminesce, but growing in large numbers in culture, or in the light emitting organs of the flashlight fish the organism emits enough light to be visible at a distance, in fact, Vibrio cultures are bright enough to read by in a darkend room.

These investigators concluded that the Vibrio was producing a substance they termed autoinducer (A1) which at low density diffused away from the cells and was in insufficient concentration to activate the luminescence mechanism (Nealson et al., 1970). At high cell density and A1 concentration the enzyme luciferase was induced and light emission initiated. Other induction mechanisms were known at the time, what was unique about this one? In other systems, such as the Lac operon described by Jacob and Monod (1961), induction is initiated by an exogenous effector molecule, lactose. In the Vibrio fisherii system, induction is initiated by an inducer molecule produced by the organism itself, thus autoinduction.
Since its first description, autoinduction has been shown to be widely distributed in an array of phylogenetically diverse bacteria and is responsible for the control of many important functions. Although the functions controlled by autoinduction are various, they have certain properties in common. These include the observation that most systems involving autoinduction occur in biofilms, that is aggregations of bacteria and other microorganisms attached to surfaces that are generally embedded in an extracellular polymeric matrix that they themselves synthesize (Table 1). Furthermore, many, although not all such autoinduction systems, operate in symbiotic, commensal, or parasitic systems in which bacteria interact with eukaryotic host organisms (Table 1).
Far from being an oddity in nature it appears that greater than 99 percent of bacteria living in nature exist in the biofilms, cells growing planktonically are they exception.
| Organism | Signaling molecule | Genes involved | Phenotype regulated |
Pseudomonas |
3OC12-AHL |
LasI/LasR |
External virulence factors: Alginate production, Type IV pili (twitching motility) elastase |
Agrobacterium |
3-oxo(octanoyl) |
traI/traR |
Conjugal transfer of the Tj Plasmid involved in Crown Gall disease |
Vibrio fischeri |
N-(3oxyhexanoyl |
IuxI/IuxR |
Bioluminescence
|
Vibrio harveyi |
N-(3-hydroxy butanoyl) Homoserine lactone |
LuxL, M, N, LuxR |
Bioluminescence |
Serratia liquefaciens |
N-butanoyl-L-homoserinelactone |
swrIgene |
Swarming motility |
Erwinia carotovora |
N-3-(oxohexanoyl)-L-homoserine, lactone |
Car gene |
Pectate lyase, cellulas, polygalaturonase, protease |
Micrococcus Xanthus |
A-factor (amino acids) |
|
Fruiting body |
Staphylococcus aureus |
RNAIII-activating protein (RAP) |
autoinducing peptide (AIP) |
Toxic exoprotein |
Perhaps the most extensively studied quorum sensing system is found in the human opportunistic pathogen Pseudomonas aeruginosa. P. aeruginosa is a serious pathogen in patients compromised by prior disease or injury, in those with implanted medical devices or undergoing mechanical ventilation and in patients with the autosomal recessive hereditary disease cystic fibrosis. In this organism, quorum sensing regulates many of the genes involved in biofilm production and virulence. Enzymes responsible for the production of cell surface products such as alginate, type IV pili, rhamnolipids, and extracellular products such as elastase, alkaline protease, hemolysin, cyanide and pyocyanin are all under autoinducer mediated regulation. Production of these compounds and virulence in P. aeruginosa both reach a maximum as the organism enters the late exponential phase of growth, when population density is highest.
In patients, this transition from a low density, usually planktonic and non-virulent life style to the high density disease related state usually occurs on tissue surfaces in aggregations called biofilms. Although it is known that autoinduction can occur in the absence of biofilm formation, as in Vibrio fischeri, for instance, it is becoming increasingly clear that many autoinduction phenomena in both gram negative and gram positive organisms are associated with biofilms.
The basic elements of the quorum sensing regulatory unit in Pseudomonas aeruginosa are two genes called lasI and lasR. lasI produces a protein (acyl-homoserine lactone synthetase) which mediates the synthesis of a specific acylated homoserine lactone (3-oxododecanoyl homoserine lactone), from S-adenosyl methionine and an acyl fatty acid (Figure 2).
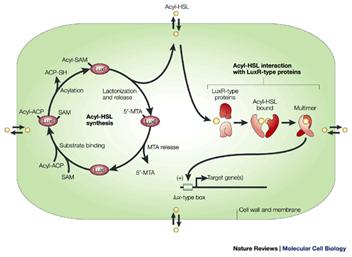
This small molecule is produced constituatively and secreted across the cell membrane. Under planktonic conditions it diffuses into the surrounding milieu and its local concentration remains low. Under conditions of high cell density as such occurs when cells are bound to surfaces, the endogenous acyl HSL concentration rises to levels approximately the same as the external concentration. At this concentration the Acyl-HSL combines with the product of the lasR gene, a protein called transcriptional activator protein. This protein is normally inactive, but when bound to this specific acyl-HSL the protein is activated and binds to the promoter regions of a number of other genes initiating their transcription. Many of these genes code for proteins involved in the development and differentiation of biofilms, these include lasA and B (elastase), apr (alkaline phosphatase) and toxA (the A exotoxin).
Subsequent investigations have revealed a second quorum sensing system called rhl which was named for the rhamnolipids produced by one of the genes under its control. As in the case of the las system there are two genes, rhlI and rhlR and each conducts an analogous function. RhlI codes for an enzyme that synthesizes an acyl-homoserine lactone (N-butyrylhomoserine lactone) which serves as an autoinducer of the rhlR transcriptional activator protein RhlR.
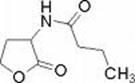
RhlR, when activated by binding to Butyryl-HSL, promotes the transcription of a number of genes including rhl A and B (rhamnolipid surfactant molecules), lasB (elastase) and possibly rpoS, the stationary phase a factor which reulates survival under adverse conditions. Many of these genes are known to be involved in the expression of virulence in P. aeruginosa.
As suggested in figure 4, the rhl system is hierarchically secondary to las in that activated LarR upregulates rhlI and rhlR. The quorum sensing system in this and other organisms is under intensive investigation and regulatory cascade of bewildering complexity is emerging.
