Quorum Sensing and Cell/Cell Signaling in Bacteria
The bacterial cell’s ability to adapt to varying microbial habitats is dependent in large part to its ability to selectively turn on or turn off genes or suites of genes as environmental conditions change. The ability to refrain from making enzymes when their function is not required saves the bacterium enormous amounts of energy. Thus selective pressure has produced in many, perhaps most, bacteria mechanisms, which permit them to estimate their cellular density and to turn on genes selectively when it is advantageous to do so, often when a sufficient number of bacteria have accumulated in a given habitat. This mechanism has come to be called Quorum Sensing.
Over the past 40 years, hundreds of papers describing Quorum Sensing in a variety of organisms and associated with a wide array of bacterial activities have been published. In retrospect though, it seems surprising that this remarkable of discovery in the field of microbiology began with a fish.
In the 1970s, scientists at Harvard University and Scripps Institute of Oceanography began to report on a unique and fascinating phenomenon. The system they were investigating was the production of bioluminescence by the marine bacterium Vibrio fischeri (then called Photobacterium). This bacterium, which lives free in the ocean, also lives in large numbers in the light emitting organs of the flashlight or lantern fish (Photoblepharon palpebratus). Living planktonically in the ocean Vibro fischeri does not bioluminesce, but growing in large numbers in bacterial culture, or in the light emitting organs of the flashlight fish the organism emits enough light to be visible at a distance, in fact, Vibrio cultures are bright enough to read by in a darkened room (Figure 1).
This light organ contains a large population of Vibrio fischeri, which emits light (bioluminescence). The light produced may be masked by the fish, by closing a black lid or curtain of tissue from the lower edge of the eye socket. The bacteria continue to produce light but the light is no longer visible.
When placed into broth culture, the organism’s growth follows a normal bacterial growth curve with lag phase followed by exponential growth. Microbiologists were intrigued by the fact that at low population densities (early log phase) the organism produced virtually no light. Later, however, as the population approached stationary phase, light production was turned on. When the investigators filtered the light producing culture to remove all bacteria and added the filtrate to early log phase cultures these cells immediately “turned on”.
The investigators concluded that the Vibrio was producing a substance they termed autoinducer (A1) which at low cell density diffused away from the bacterial cells and was in insufficient concentration to activate the luminescence mechanism (Nealson et al., 1970). At high cell density and A1 concentration the enzyme luciferase was induced and light emission initiated.
A second bioluminescent marine bacterium known as Vibrio harveyi is also known, which does not form symbiotic associations with other organisms. This organism in culture behaves much like V. fischeri and light production is also known to be under the influence of autoinduction. Interestingly, the autoinducer molecule that controls bioluminescence in V. fischeri has no effect on light production in V. harveyi and visa versa. This suggests that in nature, there is more that one autoinducer molecule. Research, to work out the complexities of this system continue and it is a fascinating story.
In the early 1970s, when Nealson, Platt and Hastings were revealing this story, few microbiologists were prepared to believe it. The idea that bacteria could somehow “estimate” their population density implied that they were in communication with one another. For a population of microbiologists raised to understand bacteria as essentially “lone rangers” even when in colonies, bacterial conversations were a bit hard to swallow.
Other gene induction mechanisms were known at the time, of course, what was unique and so difficult to accept about this one? In other systems, such as the Lac operon, described by Jacob and Monod (1961) gene transcription is initiated by an exogenous effector molecule, lactose. In the instance of Vibrio fischeri the inducer molecule is produced by the organism itself, thus, autoinduction, initiates bioluminescence.
Since its first description, the phenomenon of autoinduction has been shown to be widely distributed in an array of taxonomically diverse bacteria and is responsible for the control of many important functions. Although the functions controlled by autoinduction are various, they have certain properties in common. These include the observation that most systems involving autoinduction occur in biofilms, that is aggregations of bacteria and other microorganisms attached to surfaces that are generally embedded in an extracellular polymeric matrix that they themselves synthesize. Furthermore many, although not all, such autoinduction systems, operate in symbiotic, commensal, or parasitic systems in which bacteria interact with eukaryotic host organisms (Table 1).
| Organism | Signaling molecule | Genes involved | Phenotype regulated |
Pseudomonas |
3OC12-AHL |
LasI/LasR |
External virulence factors: Alginate production, Type IV pili (twitching motility) elastase |
Agrobacterium |
3-oxo(octanoyl) |
traI/traR |
Conjugal transfer of the Tj Plasmid involved in Crown Gall disease |
Vibrio fischeri |
N-(3oxyhexanoyl |
IuxI/IuxR |
Bioluminescence
|
Vibrio harveyi |
N-(3-hydroxy butanoyl) Homoserine lactone |
LuxL, M, N, LuxR |
Bioluminescence |
Serratia liquefaciens |
N-butanoyl-L-homoserinelactone |
swrIgene |
Swarming motility |
Erwinia carotovora |
N-3-(oxohexanoyl)-L-homoserine, lactone |
Car gene |
Pectate lyase, cellulas, polygalaturonase, protease |
Micrococcus xanthus |
A-factor (amino acids) |
|
Fruiting body |
Staphylococcus aureus |
RNAIII-activating protein (RAP) |
autoinducing peptide (AIP) |
Toxic exoprotein |
Considered an oddity when first described, autoinduction appears to be a basic control mechanism common to many bacteria.
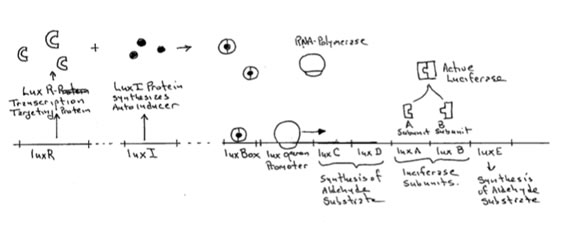
In Vibrio fischeri, light production is under the influence of three genetic elements or groups of elements, the gene luxI, the gene luxR and a group of genes called the lux operon which contains the structural genes for the production of the light-producing enzyme luciferase, and the enzymes substrate, a long chain aldehyde molecule.
-
The luxI gene contains genetic code for the production of LuxI, the protein that makes the autoinducer (3 oxohexanoyl-homoserine lactone). This compound is synthesized from S-adenosyl methionine and 3-oxohexanoyl acyl carrier protein.
S-adenosyl methionine + 3-oxohexanoyl ACP → (3 oxohexanoyl-homoserine lactone)
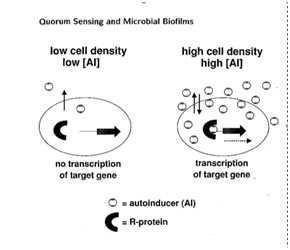 Figure 3.
Figure 3.This molecule is produced continuously and diffuses across the cell membrane. When the population of V. fischeri is low, as it is when the bacterium is living free in the open ocean, the internal concentration of the autoinducer remains low. When, however, the bacterium takes up residence in the light organ of the flashlight fish its numbers can rapidly rise to 108 or more per mm3. In the light organ, the concentration of the autoinducer rises rapidly to concentrations at which it binds to two subunits of the LuxR protein and causes the formation of an active dimer molecule initiating transcription of the lux operon.
-
Another gene, luxR codes for a protein called a transcriptional activator which when combined with VAI marks certain genes for transcription by a generalized RNA Polymerase. The active LuxR binds at a site upstream from the lux-operon called the lux box. When this site is occupied by the transcriptional activator (LuxR-dimer) RNA polymerase can bind to the lux promoter site and begin to transcribe the various genes of the lux-operon. luxC, luxD and luxE are all involved in the production of an aldehde which is the substrate oxidized by the enzyme luciferase in the production of light. luxA and luxB are the two subunits of the luciferase enzyme. When these join, the active luciferase enzyme is completed and bioluminescence commences.
-
The genes marked by the LuxR transcriptional activator are the genes of the lux operon. These consist of the two genes luxA and luxB, which code for the two subunits of the bacterial luciferase enzyme itself. The other genes, lux C, D and E code for enzymes involved in the synthesis of a long chain fatty aldehyde which is the substrate for the luciferase enzyme.
The entire system then works as shown in the following diagram (Figure 4).
Perhaps the most extensively studied quorum sensing system is found in the human opportunistic pathogen Pseudomonas aeruginosa. This organism is a serious pathogen in patients compromised by prior disease or injury, in those with implanted medical devices or undergoing mechanical ventilation and in patients with the autosomal recessive hereditary disease cystic fibrosis. In this organism, quorum sensing regulates many of the genes involved in biofilm production and virulence. Enzymes responsible for the production of cell surface products such as alginate, type IV pili, rhamnolipids, and extracellular products such as elastase, alkaline protease, hemolysin, cyanide and pyocyanin are all under autoinducer mediated regulation. Production of these compounds and virulence in P. aeruginosa both reach a maximum as the organism enters the late exponential phase of growth, when population density is highest. Why the delay? Many have speculated that P. aeruginosa, like many other opportunistic pathogens, are not particularly invasive and under normal conditions are rather easily dealt with by the host’s normal host defenses such as antibodies and phagocytic cells. If, however, the invading cells can delay the production of compounds that will trigger the hosts defense mechanisms until a substantial population of well-protected cells has matured the pathogen can in essence “fly below the hosts radar”. An attack launched once the biofilm has been formed will be resistant not only to host defenses, but to antibiotic therapy as well.
The basic elements of the quorum sensing regulatory unit in P. aeruginosa are in most respects similar to those in V. fischeri and consist of two genes called lasI and lasR (analogous to luxI and luxR. lasI produces a protein (acyl-homoserine lactone synthetase), which mediates the synthesis of a specific acylated homoserine lactone (3-oxododecanoyl homoserine lactone), from S-adenosyl methionine and an acyl fatty acid as in Vibrio fischeri.
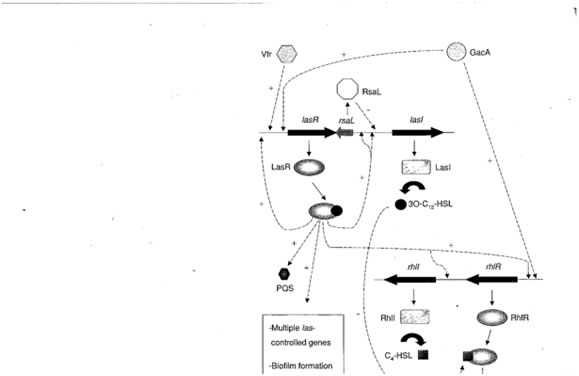
This small molecule is produced constitutively and secreted across the cell membrane. Under planktonic conditions it diffuses into the surrounding milieu and its local concentration remains low. Under conditions of high cell density as such occurs when cells are bound to surfaces, the endogenous acyl HSL concentration raises to levels approximately the same as the external concentration. At this concentration the Acyl-HSL combines with the product of the lasR gene, a protein called transcriptional activator protein. This protein is normally inactive, but when bound to this specific acyl-HSL the protein is activated and binds to the promoter regions of a number of other genes initiating their transcription. Many of these genes code for proteins involved in the development and differentiation of biofilms, these include lasA and B (elastase), apr (alkaline phosphatase) and toxA (the A exotoxin).
Subsequent investigations have revealed a second quorum sensing system called rhl, which was named for the rhamnolipids produced by one of the genes under its control. As in the case of the las system there are two genes, rhlI and rhlR and each conducts an analogous function. RhlI codes for an enzyme that synthesizes an acyl-homoserine lactone (N-butyrylhomoserine lactone), which serves as an autoinducer of the rhlR transcriptional activator protein RhlR.
RhlR, when activated by binding to Butyryl- homoserine lactone promotes the transcription of a number of genes including rhl A and B (rhamnolipid surfactant molecules), lasB (elastase) and possibly rpoS, the stationary phase a factor which regulates survival under adverse conditions. Many of these genes are known to be involved in the expression of virulence in P. aeruginosa.
|
Figure 6a. (Forthcoming)
AI-1, Butyryl Homoserine Lactone. |
Figure 6b. (Forthcoming)
AI-2, A boron containing Furanone. |
As suggested in Figure 5, the rhl system is hierarchically secondary to las in that activated LarR upregulates rhlI and rhlR. The quorum sensing system in this and other organisms is under intensive investigation and regulatory cascade of bewildering complexity is emerging.
Quorum Sensing in Gram positive bacteria.
The acyl homoserine lactones what we have looked at so far are produced almost exclusively by Gram negative bacteria, but Gram positive bacteria also exhibit quorum sensing. They just employ different auto inducers. A great variety of functions in Gram positive organisms are known to be regulated by quorum sensing, these include sporulation in Bacillus subtilus, fruiting body formation in Myxococcus xanthus, and the production of virulence factors in the pathogen Staphyococcus aureus. The basic outline of the system is much like that in Gram negative bacteria, an autoinducer is produced that accumulates with population density. At a sufficiently high concentration, the autoinducer binds to a transcriptional activator and turns on a set of genes. Perhaps the major difference is in the nature of the autoinducer itself. Unlike the Gram negative bacteria looked at so far, the autoinducers of Gram positive cells are short chain peptides (oligopeptides).
Multilingual bacteria
To this point, each of the quorum sensing mechanisms we have examined is highly individualized to the specific bacterium possessing it. It is advantageous for V. fischeri and V. harveyi to control the production of light and each has developed a quorum sensing control mechanism to do precisely that. Even though the bioluminescent result is the same the autoinducers and controlling sequences are quite different and specific to each organism.
But Bonnie Bassler at Princeton University has discovered that in addition to their “private languages”, many bacteria have developed generalized chemical messages that can be interpreted by members of other species. If the private languages like lux and las permit bacteria to assess their own population densities, these generalized languages allow them to evaluate the population densities of other bacterial populations.
Vibrio harveyi, unlike V. fischeri does not live in symbiotic relationships with eukaryotic organisms like the flashlight fish and the Hawaiian bob-tailed squid and is found typically growing as a planktonic organisms living in the open ocean, in the sediment of shallow ocean depths and on the surface and in the gut of a variety of marine animals. This organisms is also a pathogen in shrimp. None of these attributes seem to be obviously dependent on or aided by the production of light. In fact the evolutionary advantage of producing light in this organism is a matter of considerable controversy, but bioluminesce it does.
Remarkably, V. harveyi has evolved two independent systems for regulating the production of luciferase. The one is similar to that in V. fischeri in that its autoinducer is an acyl-homoserine lactone (4-hydroxyl C4 HSL) and although the receptor molecule (luxN) is not homologous to the lasR of many other Gram negative bacteria it functions autonomously as does the lux/luxR system of V. fischeri. The other quorum sensing mechanism is regulated by what Bassler terms AI-2 (autoinducer-2). This unusual signaling molecule is chemically a furanone containing boron, an element found rarely in biological systems.
The two signaling molecules, AI-1 and AI-2, are each detected by their own receptor protein, which is in turn capable of initiating the events that lead to bioluminescence. It is clear from our understanding of V. fischeri that a single cell-cell signaling molecule is sufficient to control the production of light in bacteria. Why then are there duplicate regulatory systems in V. harveyi?
Although AI-1 (Butyryl Homoserine Lactone) is found only in V. fischeri and is quite specific to that organism, AI-2, is produced by a wide variety of bacteria that are not closely related. So far, more than 30 species of bacteria are known to bear the gene, which in V. harveyi produces the furanone AI-2 and these include E. coli, S. typhimurium, Vibrio cholerae, Haemophilus influenzae, Helicobacter pylori, Bacillus subtilus, Borrelia burgdorferi, Streptococcus pneumoniae, Staphylococcus aureus and various Clostridium species. Most of these have been shown to produce the AI-2 autoinducer as well. Thus, V. harveyi can assess not only its own population density, but that of other members of its biofilm community as well. The list of bacteria producing AI-2 includes both Gram negative and Gram positive organisms and free living, and commensal as well as pathogenic organisms. This suggests that the language defined by AI-2 is widespread in nature and is used by a great variety of bacteria.
Bassler suggests that “Multilingual capabilities could enhance survival and interaction in natural habitats in which bacteria exist in mixed-species communities”. It is likely that this common language controls functions in each of the bacterial species that are important for the maintenance of the community at large. What sort of functions might these be? Costerton suggests that one such function is to control growth so that water and nutrient channels in biofilms are not closed. A coordinated message readable by all to “keep the water channels open” would be of value to the entire community.
Up to 1997, virtually all studies of quorum sensing had been carried out on a relatively few microorganisms (Table 1) under laboratory conditions. These microbial “lab rats” perform well in vitro. Genes for QS were mutated, deleted, and transferred to other bacteria in order to permit a gene-by-gene analysis of the mechanism as described. To this point however no one had demonstrated that the chemical messengers of QS (the acyl-homoserine Lactones for example) operated in natural or clinical settings. This oversight was corrected by two teams of investigators with a cross-feeding assay utilizing an AHL-responsive Agrobacterium tumefaciens reporter strain, that is the strain could respond to HSL but was unable to produce it. This bacterium had been further genetically modified by the insertion of the gene for β-galactosidase “down stream” from the Argrobacterium equivalent of lasR (called traR). Thus in the presence of any of a variety of acyl-homoserine lactones this bacterium would produce β-galactosidase.
Investigators prepared a plate of medium containing X-gal, an artificial substrate for the β-galactosidase enzyme. When this lactose analog is cleaved in two by the enzyme, a pigmented is produced, coloring the bacterium blue and indicating (reporting) the presence of β-galactosidase and acyl homoserine lactone.
McLean then placed a biofilm covered pebble from the San Marcos River (Texas) on to the plate. Subsequent examination revealed that the reporter strain of Agrobacterium was producing β-galactosidase indicating that acyl-homoserine lactone molecules on the pebble has diffused through the medium and turned on the traR locus (see Exercise 24 Detection of Naturally Occurring Acyl- Homoserine Lactones Using a Chromobacterium Reporter Strain). Pebbles that had been cleaned by sonication or subjected to autoclaving failed to stimulate the production of β-galactosidase.
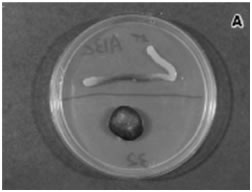
This was the first demonstration of quorum sensing signals in nature (McLean et al. 1997).
This clever experiment was the first demonstration of the presence of acyl homoserine lactones in a natural ecosystem (McLean et al.). Later (1998), members of the same team demonstrated the presence of Quorum Sensing autoinducer in biofilms infecting urinary catheters removed from patients.
We have come a long way in our journey from a marine fish that can produce light to the recognition that this phenomenon of Quorum Sensing is possibly a phenomenon common to many perhaps most bacterial species. And since we began in the ocean, perhaps it is appropriate that our story end there as well.

Some researchers examining biofilms in the shallow ocean along the south Australian Coast discovered something odd. That was, a red algae that had no biofilm at all. In a watery world where everything was coated with microorganisms this lovely algae had none at all. The algae was Delisea pulchra and the microbiologists couldn’t wait to get it back to the their lab in ______________. Into the blender it went and with the aid of a few solvents and a technique called thin layer chromatography the were able to isolate a compound called furanone. The structure of that specific furanone is shown below next to a garden variety acyl homoserine lactone. One need not be an organic chemist to see a family resemblance. It was shown that the algal furanone fits into the luxR equivalent protein of many bacteria as well as the normal autoinducer. When that site on the transcriptional activator is occupied the molecule cannot function and biofilms cannot form. If you are guessing that this caused a major commotion in the biofilm community you have gotten the point of the story. Biofilms are a major cause of chronic infections in humans, everything from pneumonia to wounds, from ear infections to the failure of implanted heart valves. As we have discussed elsewhere, these biofilms are remarkably resistant to traditional antibiotics, so once a biofilm infection in a prosthetic hip becomes established the only successful treatment is usually the removal of the device which necessitates long periods of immobility for the patient until a new one can be implanted. In instances where surgical removal of the infected site is not possible as in pneumonia, for example, the results are often fatal.
A molecule like the furanone discovered by ( ) et al. if it were available might be used to prevent the establishment of the biofilm in the first place, reducing hospital stays and cost, and saving lives.
In the event, most furanones were found to be too toxic for administration to humans. But chemists and microbiologists are working constantly to find less toxic molecules with a similar shape and action that will be, as the FDA standard says, safe and efficacious.
References
Robert.C. McLean, Marvin Whiteley, David J. Stickler and W. Claiborne Fuqua, 1997. Evidence of autoinducer activity in naturally occurring biofilms. FEMS Microbiology Letters 154: 259-263.
David J. Stickler, Nicola S. Morris, Robert J. C. McLean, AND Clay Fuqua, 1998. Biofilms on Indwelling Urethral Catheters Produce Quorum- Sensing Signal Molecules In Situ and In Vitro, Applied and Environmental Microbiology, 64: 3486–3490.



