Are there critical biofilm phenomena that diffusion does not explain?
Some other factors that may be influential
This article began with the statement that much of what makes life in a biofilm special can be explained by accounting for diffusive phenomena. Diffusion limitation probably does not explain everything though. It is likely that microorganisms have sensing pathways, which trigger gene expression during biofilm development, and that are independent of diffusion-based concentration gradients. As an approach to this issue, consider the image of an antimicrobial-treated biofilm in Figure 1. This specimen shows surviving bacteria (green) in the core of mushroom-shaped clusters that sit above a thin base film of killed (red) cells. Why are the survivors concentrated in the interior of cell clusters?
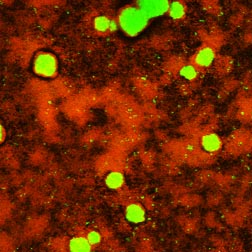
One possibility is that the antimicrobial agent failed to penetrate. From what we know about the treatment duration (240 s) and the calculated penetration time (1 s), this explanation is unlikely. Another possibility is that bacteria in the cluster interior are nutrient starved and are less susceptible by virtue of their growth state. This could be simply a matter of slow growth or it could be that "persister" cells are spawned at greater frequency in nutrient-limited environments. A third explanation is that a quorum-sensing signal accumulates in the cluster interior and triggers expression of protective genes. Certainly, all three of these hypotheses derive fundamentally from diffusion limitation. In the first case, the limitation applies to the antimicrobial agent itself, in the second case it applies to a metabolic substrate, and in the third case it applies to a metabolic product.
Can other pathways to a protected phenotypic state, which do not stem from diffusion limitation, be imagined? Perhaps microorganisms have "touch" sensors on the cell surface that mechanically detect the presence of a solid surface. Another possibility is a feedback mechanism that responds to the increased resistance to motility that must occur when a cell adheres to a surface or is neighbored by other cells and extracellular polymeric substances. Might bacteria have receptors for detecting cell-cell contact? Any of these mechanical sensing mechanisms could potentially initiate a pathway of differentiation, including the generation of cells in protected states. One of the challenges to studying such putative pathways will be demonstration that they indeed operate independent of diffusion phenomena.
Diffusion in biofilms: Summary of key concepts
Diffusion in biofilms can be summarized by the following four points:
- Diffusion is the predominant solute transport process within cell clusters.
- The time scale for diffusive equilibration of a non-reacting solute will range from a fraction of a second to tens of minutes in most biofilm systems.
- Diffusion limitation readily leads to gradients in the concentration of reacting solutes and hence to gradients in physiology.
- Water channels can carry solutes into or out of the depths of a biofilm, but they do not guarantee access to the interior of cell clusters.
Ideas for future additions to this chapter:
- Graphic: Time course of chloride ion penetration into a biofilm
- Table: De/Daq values for different types of biofilms
- Typical metabolic product profile from literature
- Inducible green fluorescent protein patterns revealing extent of oxygen limitation
- Antimicrobial penetration measurements: chlorine concentration profiles
- Antimicrobial penetration measurements: hydrogen peroxide profiles
- Full mathematical solutions for selected results
- Where a reference is cited, link to the relevant figure or image
- Simulations of transient diffusion; e.g., from cellular automata model
- Animated graphic: Electrochemical cycle that causes dissolution of metal (Section 3)
