A substantially updated version of the hypertextbook is available here. Please migrate to that version. This one will eventually disappear.
Growth and Recruitment of Additional Species
Once the nascent microcolony is established, cells of other species are recruited to the biofilm from the passing bulk fluid, which adds to the mass and the diversity of the biofilm. Some of this recruitment is random; a cell impinges on the biofilm mass “sticks” and begins to multiply. In other cases, the recruitment is very specific. Kolenbrander and his colleagues have shown that in oral biofilms cells accumulate in dental plaque in a very specific sequence much like a typical succession on a cleared field or meadow. Certain microorganisms attach to the conditioning film (pellicle) of the tooth enamel that provides receptor sites. These sites are bound by a first wave of microbes, predominantly streptococci (e.g. S. gordonii, S. sanguis and S. oralis ) from saliva, which constantly washes the enamel surface. These early colonizers are capable of binding (coaggregating) to members of their own genus (intrageneric coaggregation) and also with other genera as well (intergeneric coaggregation). This specific binding results in the rapid accumulation of a mixed species biofilm on the surface of the tooth. All of the many oral bacteria thus far tested (over 1000) bind to at least one other species. It is interesting that the bacteria engaged in binding need not be viable. Dead and metabolically inactive cells bind as readily as living ones, adding to the biofilm mass.
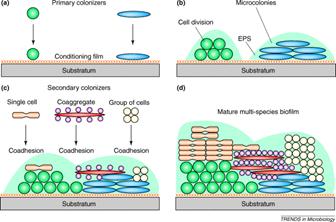
The cells of this initial wave of colonizers typically bind with actinomycetes (A. naeslundii, Fusobacterium nucleatum). Whereas the streptococci appear to have a limited range of coaggregation partners, mostly with in their own genus, the actinomycetes are capable of binding with strains of many different genera. Once they have joined the developing biofilm the species diversity increases rapidly. In a more traditional ecosystem they might be referred to as keystone species since the further development of the biofilms diversity is largely dependent on their presence.
Although originally thought to be a unique property of oral bacteria, coaggregation has been found to occur in streams, in potable-water-supplies, in the mammalian gut, in the human urogenital tract and may in fact be a general phenomenon.
In addition to cell division and aggregation, the bulk of the biofilm is increased by the accumulation of nonliving materials, silt, sand, and organics of many sorts adhere to and increase the mass and complexity of the biofilm. Some of these materials represent only structural elements while others (e.g. leaf litter) may serve as nutritive substrates for the developing biofilm.
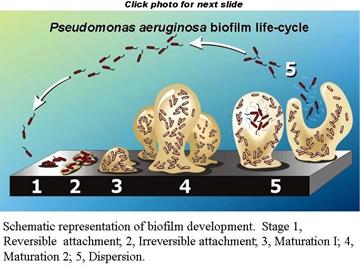
The thickness and specific architecture of the mature biofilm depends upon a number of factors including the species composition of the community, the nutrient conditions and the rate of flow of the bulk medium past the biofilm. In general, low nutrient conditions encourage robust biofilms with complex architecture while rich media result in low growing biofilms not as well attached to their substrate (Al check this, have I got this correct?). Habitats with fast, often turbulent flow (High Reynolds number) such as water mains, streams, and waterfalls result in dense biofilms often exhibiting streamers as in the classical image shown here (include Pegs drawing of the classic biofilm). Habitats with slower flow rates, and a low Reynolds number, tend to have more laminar flow and predispose to flatter less firmly attached biofilms.
Dispersal
According to R. Kolter, planktonic cells are analogous to the pioneers of human communities. They leave the aggregate and "search" for new territory to colonize. When such areas are found, these pioneers attach and a new biofilm community is established. Thus dispersal and the planktonic "lifestyle" are perhaps best seen as a transitional phase connecting one biofilm community with the next. And dispersal is the chancy, highly process of disseminating propagules into the surrounding environment each of which has only a tiny chance of "finding" a suitable spot for attachment and the founding of a new biofilm community.
Biofilms are dispersed in several ways, some of these under control of the biofilm itself and others resulting from random acts which result in the mobilization of a fragment of the biofilm. Paul Stoodley and his team have observed biofilms in a flowing environment "creeping" in waves across surfaces in the direction of flow (movie). They have also seen masses of biofilm material rolling in the direction of flow like a beech ball rolling down a grade (movie). Among the earliest forms of dispersal observed were sloughing and abrasion. In sloughing, a portion of the mature biofilm separates from its substrate and is washed downstream. The newly exposed surface is rapidly recolonized much like a plowed field or a burned over forest. This is an example of a secondary succession and in general proceeds faster than a primary succession due to the presence of biofilm remnants that form the basis of the new biofilm.
Biofilms are constantly exposed to abrasion by natural sources (water born particles, tumbling rocks, predation by bactivorous animals) or by human cleaning (scraping the hulls of ships, cleaning pipes by sending pigs through them or scrubbing out the toilet bowl). These agents or actions temporarily and locally damage the biofilm but also send large masses of biofilm further "downstream".
A more recently discovered dispersal mechanism has been described by Davies and others. In the "tower"of a mature biofilm, one may observe that the central core changes in appearance. The apparently stable core begins to roil and cells once stationary are now seen to be motile, having grown flagellae. Eventually this mass of motile planktonic cells breaks through the surface of the "tower" and disperses leaving a gaping hollow behind. This "scheduled dispersal" is under the control of chemical signals produced by the biofilm itself and is an example of Quorum Sensing mediated behavior.
