Factors Influencing Biofilm Structure
For years, debate has existed between those who feel that physical and perhaps nutritional forces are adequate to account for the physical shape of biofilms and those who are persuaded that physiological and genetic influences predominate in determining biofilm architecture. As Wimpenny notes (2000), "the truth lies somewhere between these two extremes".
It is clear that microorganisms undergo profound changes in their transition from planktonic (free-swimming) to cells that are part of a complex, surface-attached community. These changes are reflected in new phenotypic characteristics developed by bacteria and occur in response to a variety of environmental signals (O’Toole, Kaplin and Kolter 2000). In addition, recent research had demonstrated that many genes, controlling structural and enzymatic elements of the biofilm as well as many that control regulatory functions are involved in this complex transition.
Thus there are a number of identifiable factors that have been demonstrated to control or modify biofilm structure and these include hydrodynamics, nutrition, and genetics, including cell-cell signaling.
Hydrodynamics
Biofilms growing in lotic, or flowing environments like a stream, pipeline, a heart valve or your mouth are subject to hydrodynamic action which can significantly affect their structure. The mechanical force that moving liquids exert, as it moves past a surface is called fluid shear. Although shear exists throughout the fluid in biofilms, the term ‘shear stress’ is usually used in the context of the shear exerted at the solid surface, for example, where the biofilm is growing. The shear stress will tend to ‘wash away’ the attached biofilm from the surface on which it is growing and it increases as the flow rate is increased. Erosion due to shear also increases with the thickness of the biofilm.
We could use a diagram here showing the effects of fluid stress on shearing, rolling, rippling and sloughing. I am pretty sure Peg has such an image. But I also remember a video clip of a biofilm in a flow cell showing a long stramer waving in the flow like a flag. Does any one know where this is?
Shearing is the continuous removal of small amounts of biofilm at the liquid/biofilm interface as opposed to sloughing which is the sudden and massive loss of a segment of biofilm generally right down to the substratum surface. In low shear environments like the dripping walls of your shower, biofilms typically grow as isolated micro-colonies, often irregular in shape with little or no indication of direction of flow. It is here that one sees the iconic mound shaped and mushroom shaped structures pictured in the classic biofilm illustrations.
Biofilms growing in high shear environments, such as steams, water lines or a shoreline exposed to wave action tend to be denser. They also tend to form long filaments or streamers pointing in the down stream direction. These biofilms possess a characteristic called viscoelasticity that is they are flexible and stretch out when flow rate is high and contract once again when the fluid velocity is reduced.
These streamers also wave back and forth in the stream flow much like a flag in the breeze. Interestingly enough, there are other systems in which a flowing medium (air) acts on masses of denser material to produce this streamer-like appearance. Figure 1 shows an anvil shaped cloud shaped by the action of moving air on a mass of moisture saturated air (cloud), also forms a streamer which resembles the streamers of biofilms in a flowing environment.

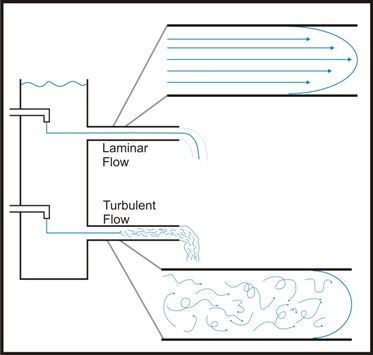
The flow of a liquid can be described as either laminar or turbulent. The value used by engineers to measure this property is called Reynold’s number. Engineers calculate Reynold’s number as a number with no physical dimensions calculated as a function of the density, viscosity, linear distance traveled and velocity of a flowing fluid. It can be used to describe the hydrodynamics of a flowing system. In flowing systems with a low Reynold’s number (Re), laminar flow may exist which implies that a given "plug" of liquid moves through the system (e.g. a pipe) with little or no mixing. In turbulent flow the liquid in the plug is rapidly mixed. Laminar flow is usually characterized by low fluid velocity (Re < 2100) while high fluid velocity generally leads to turbulent flow (Re > 4000).
The velocity of the water near the wall of the channel is lower than that closer to the center of the channel. This is due to the interaction between the solid surface of the channel wall and the viscous nature of the water. This area of altered velocity is referred to as the hydrologic boundary layer. In a laminar flow environment this boundary may make it difficult for bacteria to actually reach the surface and may account for the observation that mutants of motile bacteria lacking flagellae are far less efficient in forming biofilms that wild type cells.
The water very near the surface of a flow cell in laminar flow may be moving so slowly that motile bacteria can even swim upstream even though the velocity of the bulk fluid at midstream may be as much as 10 cm sec-1.
In natural systems, turbulent flow is the rule. Uneven surfaces, air bubbles, suspended particulates and many other factors tend to make laminar flow unlikely even at relatively low fluid velocity. Under these conditions, cells are usually brought to the surface by fluid dynamic forces and cell motility is of considerably less importance. Although a boundary layer exists in these conditions in which the flow is nearer to laminar, this layer can be penetrated by the turbulence delivering bacteria and nutrients to the channel surface.
Once cells have become irreversibly attached to the channel wall, their subsequent growth and development is at least in part dependent on the hydrodynamics of the system. Biofilms grown under laminar flow, tend to be thicker, but less dense than those grown under turbulent flow conditions.
Nutrition
It has been shown that biofilms will grow on surfaces in nutrients solutions so dilute that they will not support the growth of planktonic cells. This observation supports the contention that biofilm acts as an adsorbent layer concentrating organic materials and other nutrients from the bulk fluid. High nutrient concentrations tend to produce biofilms that are thicker and denser than those grown in low nutrient concentrations.
The nutritional requirement for biofilm formation appears to be rather species specific. O’Toole reports, for example that P. aeruginosa and P. fluorescens will produce their characteristic biofilms on almost any nutrient conditions that will support growth but that E. coli and Vibrio cholera will do so only if supplemented with various amino acids. The enterotoxigenic strain of E. coli O157:H7 is quite fussy and will form biofilms only on media lacking all but the poorest nutrients (O’Toole et al. 2000).
Paul Stoodley’s lab demonstrated that a mature mixed species biofilm could change its morphology from ripples and streamers to densely packed mound-like structures when the nutrient concentration was increased ten fold (Stoodley et al. 1999c). These more densely packed biofilms had a greater tendency to slough off the substrate surface than did the ripples and streamers formed at lower concentration.
At the other extreme, however, Stoodley also showed that the rate of detachment from the substrate in an Aeromonas hydrophila biofilm was also increased by nutrient limitation. So perhaps either too much or too little nutrient predisposes to detachment.
It seems clear however that it is more than simply the nutrient concentration that influences the architecture of a mature biofilm. Klausen et al.. 2003 showed that different carbon sources greatly affected the structure of biofilms in Pseudomonas aeruginosa PAO1. Glucose grown biofilms produce the tower and mushroom shapes separated by water channels typical of this organism. When grown under the same conditions with citrate substituted for glucose, however, the biofilms formed by PAO1 are flat, homogeneous and lack evidence of water channels.
Costerton paints an image of planktonic bacteria oxidizing substrates and extruding protons via electron transport mechanisms across their cell membranes into the external environment (Mitchell and Moyle 1965). He postulates that this would work well in a nutrient rich low flow environment where protons might accumulate to sufficient concentrations so as to reenter the membrane with subsequent ATP production by oxidative phosphorylation. In a high flow, turbulent environment these protons would rapidly be lost to the cell of group of cells producing them. In such an environment, cells encased in a matrix rich biofilm would be as a significant advantage.
Nutrients, even those in low concentration, delivered to the biofilm in the bulk fluid through the complex system of water channels would be adsorbed into the biofilm matrix where they become available to the cells. Costerton further argues that this efficiency of energy gathering and utilization might have been a major selective advantage in the evolution and dominance of biofilms particularly in turbulent ecosystems such as mountain streams where nutrient concentrations are low and contact between nutrients and cells is transient.


