Chapter 11 Lab Exercises
Section 13 Measurement of Biofilm Thickness
Page 3 Student
Copyright © Alfred B. Cunningham, John E. Lennox, and Rockford J. Ross, Eds. 2001-2010
Measurement of Biofilm Thickness
Student Version (go to Instructor Version)
Supplies Needed:
|
Quantity |
Description |
| 1 | glass cover slip |
| 1 | felt tip marker |
| 1 | microscope - modified to make depth measurements |
| 1 | metric feeler gauge - used to calibrate the
microscope (optional) |
Introduction
The thickness of biofilms is an important parameter for scientists and engineers to know because it is related to the rate of growth of the biofilm and the extent to which biofilms interfere with human engineered devices. The rate at which nutrients and antimicrobics penetrate biofilms is related to their thickness, as is the rate to which they respond to antimicrobics like disinfectants and antibiotics. The degree to which biofilms interfere with the fouling of pipes and fluid delivery systems and interfere with the function of devices like heat exchangers is also due, in part, to the depth of the biofilm (see Figure 1).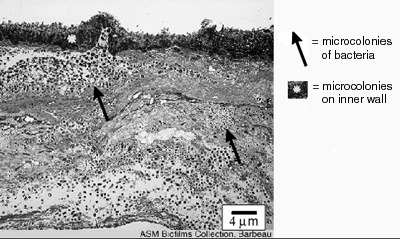
Figure 1. This biofilm cross section was obtained
with electron microscopy from the interior wall of a
dental office evacuation system. The biofilm shown is
approximately 36 µm thick.
Instructions
In this exercise you will use a modified microscope to measure the depth of a biofilm. Notice that the microscope has a piece of polar coordinate graph paper fixed to its base surrounding the fine and course adjustment knobs. Attached to the fine adjustment knob is a thin piece of wire that serves as a pointer.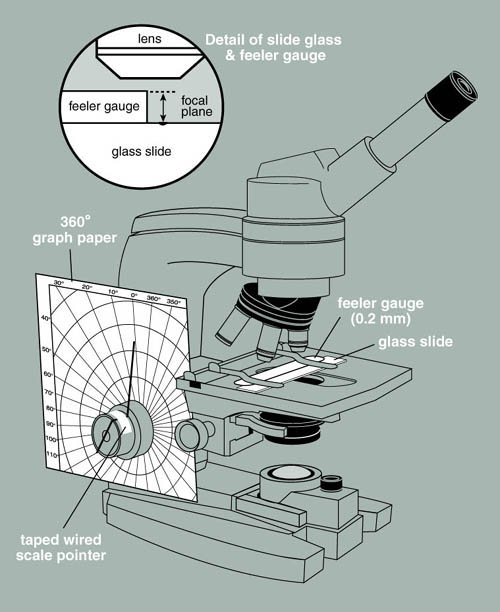
Figure 2. A microscope modified to measure
depth.
Calibrating the microscope to measure depth
Your instructor will provide you with a metric feeler gauge normally used by automobile mechanics and engineers to measure the gap between two points (spark plug gap for example). The leaves of this gauge are precisely machined and are very delicate, so "handle with care."- With transparent tape, fix the 0.04 mm feeler gauge tightly to the surface of a microscope slide. Along one edge of the gauge, on the slide, make a mark with a glass marking pen. This will make it easier to focus the microscope on the surface of the slide.
- Mount the slide with feeler gauge attached into the mechanical stage of your microscope and focus on the top surface of the metal gauge at the edge closest to the mark you made earlier.
- When the microscope is in focus on the gauge, record
the angle in degrees shown by the pointer on the polar
coordinate graph paper.
Top of gauge = ___________ degrees. - Now, carefully focus down so that the microscope is in
focus on the top surface of the microscope slide. Look for
the mark you made earlier. Record this angle.
Top of slide = _____________ degrees.
Determine the difference in degrees arc between these two readings.
We now have the depth of the feeler gauge (0.04mm or 40 µm) in terms of degrees of arc from the graph paper. If for example, 82 degrees of arc moves the lens from the surface of the gauge to the surface of the slide (82 degrees of arc = 0.04 mm or 40 µm; therefore 1 degree of arc = 0.49 µm or approximately 0.5 µm).
With this simple device, you can now measure the thickness of living biofilms non-destructively over time.
Biofilm thickness measurement
- Place the biofilm (either on a glass microscope slide
under a coverslip or in a flow cell, on the stage of the
microscope. Focus on the very top of the biofilm and record
the reading of the pointer on the graph paper.
Top of biofilm = ___________ degrees.
- Carefully focus on the substrate level (base of the
biofilm) and record that reading as well.
Base of biofilm = ___________ degrees.
- The thickness of the biofilm is the difference is
degrees of arc times the length value you have determined
for one degree of arc through your calibration.
Thickness of biofilm = ___________________.
Refractive index correction
- From your earlier experiences in microscopy you have studied the concept of refractive index. You will recall that different transparent materials have different Indices of Refraction (n). In practice this means that as light passes through these materials it bends to different degrees. Air has about the same index of refraction as the vacuum of space and is set as the standard with an n of 1.00. Water on the other hand has an n of 1.33. So light passing from air into water is bent. You have probably observed this phenomenon while observing a stick placed into water. At the air/water interface the stick appears to bend.
- This same concept implies that the depth of the biofilm you have measured (optical thickness) is not quite accurate and to get the "real" length or physical length, a correction factor is required (See Figure 3 and Reference).
-
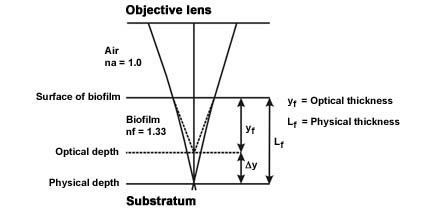 Figure 3. Light paths from the substratum-biofilm interface, through the biofilm sample and air to the objective lens.
Figure 3. Light paths from the substratum-biofilm interface, through the biofilm sample and air to the objective lens.
(Modified from Bakke and Olsson, see reference) - The physical length Lf ≈ kfyf where yf = the optical thickness measured and kf = a proportionality constant function of the refractive indices of the biofilm and air.
- Since the proportionally constant kf ≈ nf / nf (Refractive index of the biofilm [nf = 1.33 or approximately that of water] divided by the refractive index of air [nf = 1.0]) the proportionality constant = approximately 1.33.
- The actual length (Lf ≈ kfyf) is therefore the measured optical distance (yf) multiplied by the constant 1.33 (kf).
References
Biofilm Thickness Measurements by Light MicroscopyR. Bakke and P.Q. Olsson
J Microbiological Methods 1986; 5:93-98.
Educational Program Curricula and Teaching Resources
Supported in part by the Waksman Foundation for MicrobiologyDeveloped in collaboration with Dr. John Lennox, Penn State University-Altoona
©1999-2008 Center for Biofilm Engineering, http://www.biofilm.montana.edu
