Detection of Naturally Occurring Acyl- Homoserine Lactones Using a Chromobacterium Reporter Strain
Student Version (go to Instructor Version)
CONTENT
Many functions in bacteria are regulated by a phenomenon variously called autoinduction or quorum sensing (QS). In QS, the bacteria constantly produce small highly diffusible molecules (often but not always acyl-homoserine actones [acyl-HSLs]), which cross the plasma membrane to the external environment. At low levels these molecules produce no evident effect. As population density increases or as diffusion is constrained, as in a biofilm for example, the concentration of A-HSL rises. At some threshold level the A-HSLs bind reversibly to specific m-RNA synthetase enzymes and these commence transcription of suites of genes previously inactive.
When first described as the mechanisms responsible for controlling bioluminescence Vibrio fisherii, autoinduction was thought a quaint if interesting phenomenon1. This view changed rapidly as A-HSLs were identified as the effector molecules2 and were shown to be functioning in a great number of additional bacterial systems including virulence3, motility4, pigment production in some bacteria5, and the develoment of normal biofilm architecture6, among many others.

The cartoon shown in Figure 1 illustrates the concept of bacterial quorum sensing. In suspension, bacterial cells produce homoserine lactone (HSL) signals, but the signal concentration is so low that bacterial activity remains unaffected. In the relatively tight quarters of a biofilm community, or a colony growing on a plate, the HSL concentrations can be locally elevated, increasing both the external and internal concentrations of HSL. When concentrations are high enough, HSL attaches to HSL receptors. In the case of Chromobacterium violaceum, this triggers pigment formation.
In 1997, McLean et al.7 demonstrated the presence of A-HSLs in naturally occurring biofilms growing on limestone pebbles taken from the San Marcos river in Texas. Their original report employed a strain of Agrobacterium tumifaciens that incorporated a Lactose operon borne on a plasmid. For technical reasons this strain is difficult to maintain in laboratory culture and requires antibiotics to maintain the plasmid. Fortunately other investigators have developed a different reporter system using violacein production in Chromobacterium violaceum5 that overcomes these problems.

Wild type Chromobacterium violaceum is a purple pigmented organisms due to the production of violacin. C. violaceum strain CV026 fails to produce pigment due to the inability to produce the normal autoinducer N-hexanoyl HSL. Although normally non-pigmented, this strain responds to the presence of exogenous HSL by producing normal amounts of the violet pigment. C. violaceum strain 31532 over-produces the N-hexanoyl HSL, but fails to respond to it and is thus is also non-pigmented while the wild-type strain 12472 both produces and responds to N-hexanoyl HSL by producing violacein.
INSTRUCTIONAL OBJECTIVE
When you have completed this exercise you will have a firmer grasp of the concept of Quorum Sensing and should be able to explain the various components of a QS cascade. In addition the techniques learned, may enable you to formulate and carry out your own research program using the organisms provided and the new skills you have learned.
BACTERIAL STRAINS USED IN THIS INVESTIGATION
| Strain Designation | Description | Properties |
|---|---|---|
| ATCC 12472 | Wild Type (pigmented) | Produces N-hexanoyl-HSL (C6-HSL) and responds to same. |
| CV 026 | Reporter Strain (non-pigmented) | Fails to produce C6-HSL, but does respond to it. |
| ATCC 31532 | C6-HSL Producer (non-pigmented) | Over produces C6-HSL, but does not respond to it. |
MATERIALS REQUIRED
| Quantity | Description |
|---|---|
| 1 culture | Chromobacterium violaceum Strain CV026 (reporter strain). |
| 1 culture | Chromobacterium violaceum Strain 31532 (over-producer of N-hexanoyl HSL). |
| 1 culture | Chromobacterium violaceum Strain 12472 (wildtype). |
| As Necessary | 3 plates of LB or R2A medium per student. |
| As Necessary | Source of biofilms (pebbles from streams, soil, aquarium samples, etc.). |
EQUIPMENT PER STUDENT
| Quantity | Description |
|---|---|
| 1 | Zip-loc plastic bag for collection samples |
| 1 | Sterile glass Petri dish |
| 1 | Pair forceps |
| 1 | Disinfectant for lab bench |
INSTRUCTIONAL PROCEDURES
Prior to the laboratory period but within 24 hours
- Collect and return to the laboratory samples suspected of containing naturally occurring biofilms, pebbles from streams, soil samples, scrapings from aquaria, material from sink traps, etc. Divide samples in two equivalent sets and autoclave one sample set to serve as a negative control. Alternatively, the rock can be sonicated and washed in sterile distilled water to remove the biofilm.
During the Laboratory Period
- Using the techniques you have learned in previous laboratory sessions, streak the three plates as shown in the following diagrams.
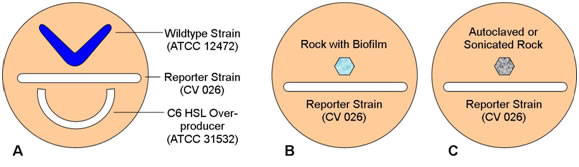 Figure 1.
Figure 1.
- Remember that strain 12472 is a wildtype which both produces and responds to N-hexanoyl HSL, strain CV026 responds to but does not produce N-hexanoyl HSL, and strain 31532 is an overproducer of N-hexanoyl HSL but does not respond to its presence. What pattern of pigment formation do you predict will occur in each plate?
- Incubate the plates at room temperature until the next lab period.
- Using the diagram shown here, indicate where pigment formation has occurred.
 Figure 2.
Figure 2.
- Interpretation: How do you interpret these results?
QUESTIONS
- Describe differences and similarities between Quorum Sensing as
illustrated by pigment production in Chromobacterium violaceum and gene requlation as described in the Jacob and Monod model.
- If in your experiment with the rock containing a biofilm you observed no results, does this imply that no QS phenomena are taking place in the biofilm bacterial population? Explain.
- Spend a few minutes on the Internet and see how many other bacterial systems are known to be under the control of QS type systems in addition to pigment production.
FOLLOW-UP ACTIVITIES
This assay system can be used as the starting point for any number of student generated research protocols.
- Individual bacterial strains isolated from nature can be tested for the production of C6-HSL.
- It has been demonstrated that other Acyl Homoserine lactones may interfere with the response to the native C6-HSL and cause a failure of pigment production in the wildtype strain (ATCC 12472). This phenomenon has been referred to as Quorum Sensing suppression. Bacterial strains and natural products (Herbs, spices, fruit juices, antimicrobials and many other materials can be tested for QS suppression.
- Ethyl acetate extracts of bacterial strains can be subjected to Thin Layer Chromatography and these TLC plates can then be overlaid with soft agar suspensions of the reporter strain to detect C6-HSL of the wild type strain, to detect other HSL secies by Quorum Sensing suppression.
- Another detection system using an Agrobacterium tumefaciens strain bearing a Lac operon can be used to detect a wider range of Acyl-HSLs. (See McClean R.J.C. et. al, 1997).
REFERENCES
- Nealson, K.H., Platt, T. and Hastings, J.W., 1970. Cellular Control of the Synthesis and Activity of the Bacterial Luminescent System. Journal of Bacteriology 104: 313-322.
- Fuqua, W.C., Winans, E.P., and Greenberg, E.P. 1994. Quorum Sensing in Bacteria: the Lux R-Lux I Family of Cell Density-Responsive Transcriptional Regulators. Journal of Bacteriology 176: 269-275.
- Passador, L., Gambello, M.J., Rust, L., and Iglewski, B.H. 1993. Expression of Pseudomonas aeruginosa Virulence Genes Requires Cell-to-cell Comunication. Science 260: 1127-1130.
- Hentzer, M., Givskov, M., and Eberl, L. 2004. Quorum Sensing In Biofilms: Gossip in Slime City. IN Microbial Biofilms, Ghannoum, M., and O’Toole, G.A., Eds.
- McClean, K.H., Winson, M.K., Fish, L., Taylor, A. Chhabra, S.R., Daykin, M., Lamb, J.H., Swift, S., Bycroft, B.W., Stewart, G.S.A.B., and Williams, P., 1997. Quorum Sensing and Chromobacterium violaceum: Exploitation of Violacein Production and Inhibition for the Detection of N-acylhomoserine lactones. Microbiology 143: 3703-3711.
- Davies, D.G. Parsek, M.R., Pearson, J.P., Iglewski, B.H., Costerton, J.W., and Greenberg, E.P. 1998. The Iivolvement of Cell-to-cell Signals in the Development of a Bacterial Biofilm. Science 280: 295-298.
- McClean, R.J.C., Whiteley, M., Stickler, D.J., and Fuqua, W.C., 1997. Evidence of Autoinducer Activity in Naturally Occurring Biofilms, FEMS Microbiology Letters 154: 259-263.
This material is based upon work supported by the National Science Foundation under Grant No. 0618744. Developed in collaboration with Dr. John Lennox, and Penn State Altoona and Robert McLean, Texas State University, San Marcos TX. Any opinions, findings, and conclusions or recommendations expressed in this material are those of the authors and do not necessarily reflect the views of the National Science Foundation ©2002-2008 Center for Biofilm Engineering, http://www.biofilm.montana.edu
