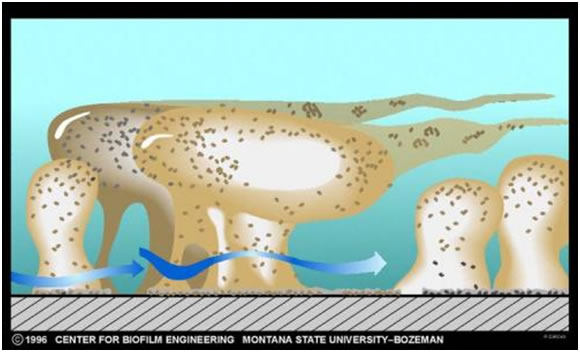
Growth and Recruitment of Additional Species
Once the nascent microcolony is established, cells of other species are recruited to the biofilm from the passing bulk fluid, which adds to the mass and the diversity of the biofilm. Some of this recruitment is random; a cell impinges on the biofilm mass “sticks” and begins to multiply (Figure 1). In other cases, the recruitment is very specific.
Kolenbrander and his colleagues have shown that in oral biofilms cells accumulate in dental plaque in a very specific sequence much like a typical ecological succession on a cleared field or meadow (see Exercise 22: Coaggregation: Interactions between Bacteria in Dental Biofilms). Certain microorganisms attach to the conditioning film (acquired pellicle) of the tooth enamel that provides receptor sites. These sites are bound by a first wave of microbes, predominantly streptococci (e.g. S. gordonii, S. sanguinis and S. oralis ) from saliva, which constantly washes the enamel surface. These early colonizers are capable of binding (coaggregating) to members of their own genus (intrageneric coaggregation) and also with other genera as well (intergeneric coaggregation). This specific binding results in the rapid accumulation of a mixed species biofilm on the surface of the tooth. The most common sort of binding is between a lectin (carbohydrate binding protein) on one cell and a specific carbohydrate sequence on the other (more of this in Chapter 5). All of the many oral bacteria thus far tested (over 1000) bind to at least one other species. It is interesting that the bacteria engaged in binding need not be viable. Dead and metabolically inactive cells bind as readily as living ones, adding to the biofilm mass.
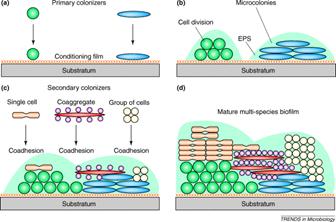
The cells of this initial wave of colonizers typically bind with actinomycetes (A. naeslundii),and Fusobacterium nucleatum). Whereas the streptococci appear to have a limited range of coaggregation partners, mostly within their own genus, and members of the actinomycete group, the fusobacteria are capable of binding with strains of many different genera. Once the fusobacteria have joined the developing biofilm the species diversity increases rapidly. In a more traditional ecosystem they might be thought of as keystone species (glossary) since the further development of the biofilms diversity is largely dependent on their presence. Figure 1 shows a rod-shaped or ilamentous bacterium, perhaps Fusobacterium, coaggregated with a number of cocci, probably Streptococcus in an interesting aggregation called a “corncob”. See exercise entitled Coaggregation: Interactions between Bacteria in Dental Biofilms.
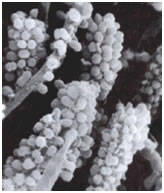
Although originally thought to be a unique property of oral bacteria, coaggregation has more recently been found to occur in streams, in potable-water-supplies, in the mammalian gut, in the human urogenital tract and may in fact be a general phenomenon in lotic or flowing environments.
In addition to cell division and aggregation, the bulk of the biofilm is increased by the accumulation of nonliving material. Silt, sand, and organics of many sorts adhere to and increase the mass and complexity of the biofilm. Some of these materials represent only structural elements while others (e.g. leaf litter, chitin) may serve as nutritive substrates for the developing biofilm.
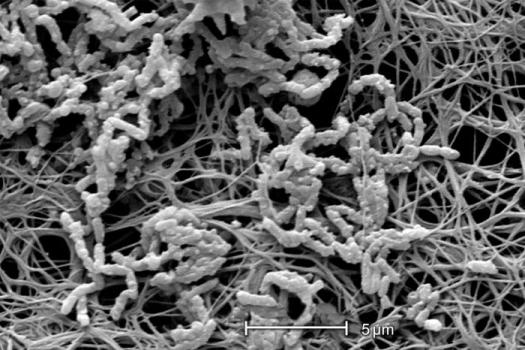
The thickness (see exercise entitled Measurement of Biofilm Thickness) and specific architecture of the mature biofilm depends upon a number of factors including the species composition of the community, the nutrient conditions and the rate of flow of the bulk medium past the biofilm. In general, low nutrient conditions encourage robust biofilms with complex architecture while rich media result in low growing biofilms not as well attached to their substratum. Habitats with fast, often turbulent flow (High Reynolds number) such as water mains, streams, and waterfalls result in dense biofilms often exhibiting streamers as in the classical image shown in Figure 3. Habitats with slower flow rates, and a low Reynolds number, tend to have more laminar flow and predispose to flatter less firmly attached biofilms.