A Brief Introduction to Biofilms
Biofilm in pipe section. |
Biofilm in stream in Yellowstone National Park. |
Biofilm scraped from reverse osmosis membrane. |
Even though you may not be familiar with the term biofilm, you have certainly encountered biofilms on a regular basis. For example, the plaque that forms on your teeth and causes tooth decay is one type of bacterial biofilm. The "gunk" that clogs your household drains is also a biofilm. If you have ever walked in a stream or river, you may have slipped on rocks that were slimy with biofilm. A persistent infection on a scrape you got from a sports injury was likely a biofilm. And so it goes: biofilms—they're where you want to be.
So what is a biofilm?
A biofilm is composed of living, reproducing microorganisms, such as bacteria, that exist as a colony, or community. In other words, biofilms are alive and have a complex social structure that scientists and engineers are still trying to unravel, a structure that both protects them and allows them to grow.
How do biofilms form?
A biofilm forms when certain microorganisms (for example, some types of bacteria) adhere to the surface of some object in a moist environment and begin to reproduce. The microorganisms form an attachment to the surface of the object by secreting a slimy, glue-like substance. Biofilms can form on just about any imaginable surface: metals, plastics, natural materials (such as rocks), medical implants, kitchen counters, contact lenses, the walls of a hot tub or swimming pool (did you ever notice that the sides of a hot tub or swimming pool seemed slightly slimy?), human and animal tissue, and on and on. Indeed, wherever the combination of moisture, nutrients, and a surface exists, biofilms will likely be found as well.
A biofilm community can be formed by a single kind of microorganism, but in nature biofilms almost always consist of mixtures of many species of bacteria, as well as fungi, algae, yeasts, protozoa, and other microorganisms, along with non-living debris and corrosion products. For example, over 500 bacterial species have been identified in typical dental plaque biofilms!
How big can a biofilm get?
Biofilms can be so thin as to avoid detection by the naked eye—just a few cell layers thick. The biofilms that almost certainly exist on your kitchen counter, for instance, are generally undetectable to the eye (unless, like some college students, you don't wash your counters very often). They can also grow to become many inches thick; probably not on a countertop (at least we hope not), but certainly as algae on rocks in a streambed.
What makes a biofilm stick together?
Engineers and scientists have discovered that biofilms are held together by sugary molecular strands, collectively termed "extracellular polymeric substances" (a mouthful of a term that essentially means "compounds or substances that form outside the walls of cells") or "EPS." The cells produce strands of EPS and are held together by these strands, allowing them to develop complex, three-dimensional, attached communities that are resistant to attacks that would destroy individual cells not part of a biofilm colony.
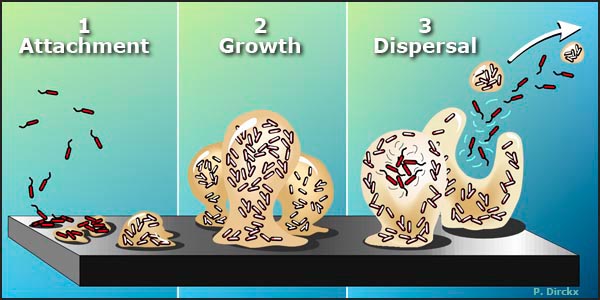
Figure 1. The Biofilm Life Cycle.
1. Free-floating, or planktonic, bacteria encounter a submerged surface and within minutes can become attached. They begin to produce slimy extracellular polymeric substances (EPS) and to colonize the surface.
2. EPS production allows the emerging biofilm community to develop a complex, three-dimensional structure that is influenced by a variety of environmental factors. Biofilm communities can develop within hours.
3. Biofilms can propagate through detachment of small or large clumps of cells, or by a type of "seeding dispersal" that releases individual cells. Either type of detachment allows bacteria to attach to a surface or to a biofilm downstream of the original community.
How can harmful biofilms be treated?
Ah! Now here we get to the crux of the biofilms issue. Read this little section carefully, because when you get the point here, you will understand why the study of biofilms is so radical and important, and the rest of this hypertextbook will make sense to you. You see, the effective treatment (i.e., destruction) of harmful microorganisms, such as bacteria, has been studied for many long years from the wrong perspective! That's right. Harmful microorganisms were studied (and still are, unfortunately, to a large extent) in isolation, not as members of a biofilm colony, where they actually normally reside.
Let's discuss this by way of an example. Have you ever heard of, say, a mouthwash that is advertised to "kill millions of germs on contact?" That is probably a truthful statement from the mouthwash company (hey, we aren't trying to run anyone out of business; however, we would like to help them do their business better). But now consider this. Those millions of germs are embedded in a plaque biofilm in your mouth. Just how does the mouthwash penetrate the biofilm to actually contact each of those millions of individual germs? Good question. Remember that we said that biofilms can become quite thick, and that they seem to have the capacity to form defensive mechanisms against outside attacks.
The fact is that today's mouthwashes, antibiotics, household cleaners, disinfectants, and most other treatment formulas were developed by testing their effect on harmful microorganisms in a planktonic state (a state in which microorganisms float in a solution as individuals, not as part of a biofilm colony attached to a surface). So, although such treatments may indeed kill millions of germs on contact, the effect of these treatments is severely limited when they are applied to real world environments in which the microorganisms to be killed are actually members of a biofilm.
The conclusion, and one that is currently being aggressively pursued by biofilm researchers around the world, is that the entire study of the treatment of harmful microorganisms must be revisited in the light of this new understanding of how those microorganisms actually present themselves in our environment: as biofilms. The structure, growth, defense mechanisms, reproduction, and all other facets of biofilms must be understood, and new treatments and methods for evaluating the effectiveness of those treatments must be developed.
These are the grand challenges facing those who enter the field of biofilms. Much progress has already been made, but there is a lot more to understand and do, both in terms of developing effective treatments and in educating society about biofilms and their implications. That is why we say that this is a good field for young people to consider as a career focus in biology, microbiology, environmental engineering and science, biochemistry, and other disciplines.
Oh, and let's finish the story about the mouthwash. Given what is known about dental plaque now, you would have to gargle with a good mouthwash for about five minutes straight to kill off those millions of germs present on teeth coated with an "average" amount of plaque.
What are the Financial Impacts of Biofilms?
Biofilms cost the U.S. literally billions of dollars every year in energy losses, equipment damage, product contamination and medical infections. But biofilms also offer huge potential for bioremediating hazardous waste sites, biofiltering municipal and industrial water and wastewater, and forming biobarriers to protect soil and groundwater from contamination. The complexity of biofilm activity and behavior requires research contributions from many disciplines such as biochemistry, engineering, mathematics and microbiology. New insights into the mysteries of biofilm are being published regularly in a wide variety of science and engineering journals.





