Objectives
The objectives of this section are
to provide you, on the one hand, with a few more detailed examples how biofilms can be used in beneficial ways;
to give you, on the other hand, a few more detailed examples of some of the harmful impacts of biofilms.
Outcomes
Upon completion of this section, you will be able
to discuss more detailed examples of both the beneficial and harmful impacts of biofilms on our world;
to describe in an informal way how the biofilm mechanism works to achieve either a beneficial or harmful effect, based on the situation in which the biofilm appears.
How do Biofilms Impact Our World?
About This Section
In the section "Where do Biofilms Grow?" you learned that biofilms form and grow in practically every possible environment on earth. That being the case, what is their impact on the earth? Can we use them for beneficial purposes? How do they affect human life? We explore these questions here.
From the previous sections you have learned that biofilms:
- are a natural and important part of our world.
- are found virtually everywhere on earth, including in extreme environments.
- are an integral part of the human body.
- can be quite harmful to human health.
- cause industry all sorts of problems and expense.
- have beneficial uses as well as harmful impacts.
In this section we explore some of these effects of biofilms in more detail. Remember as you read this section that although we speak of biofilms as the collective term for the issue we are exploring, there are many, many different kinds of biofilms, each made up of communities of different microorganisms, which is why some can be good and others harmful.
Some examples of the beneficial and detrimental aspects of biofilms are summarized below.
Beneficial Biofilms
In natural environments
As we have already pointed out, biofilms are all around us, on us, and in us. Obviously, then, not all biofilms are harmful. Many play an important role in the ecology of the earth and the sustainability of life in general. The report, "Global Environmental Change: Microbial Contributions, Microbial Solutions," points out: ". . .the basic chemistry of Earth's surface is determined by biological activity, especially that of the many trillions of microbes in soil and water. Microbes make up the majority of the living biomass on Earth and, as such, have major roles in the recycling of elements vital to life." Imagine that! "Microbes make up the majority of the living biomass on Earth," and, as we are learning, those microbes often live in biofilm colonies on surfaces.
For example, it is known that bacteria are early colonizers (in a biofilm) of initially clean surfaces submerged in water. Scientists have been able to document a predictable pattern of the way in which biofilms form on a clean surface under water. Whether the surface in question is a boat hull floating on top of the water, or a new deep sea vent at the bottom of the ocean, microbes are already present in these environments and are capable of rapid attachment to these surfaces. From these pioneers, development as a biofilm rapidly commences.
It is important to recognize that microorganisms, such as bacteria, that colonize in biofilms have evolved along with other organisms, including human beings. While some bacteria produce effects that are bad for other organisms, most bacteria are harmless or even beneficial. When it comes to bacteria, higher organisms (like us) are just another environment to colonize. So here's a thought: humans, who are often thought to be the colonizers of the world, are themselves the target of colonial powers, in the form of the many microorganisms that sneak into and inhabit our body!
Water and wastewater treatment
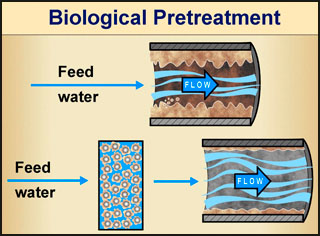
One of the best examples of successful, beneficial application of biofilms to solve a huge problem is in the treatment of wastewater. Think of it this way. We know that microorganisms are the main agents that cause decay in dead plants and animals. Decay happens (partly) as the microorganisms feed on the tissue of the dead organism. Since that is true, perhaps one could engineer a system that uses the proper microorganisms (in the form of a biofilm) to process wastewater and sewage: if the contaminated water were passed through such a biofilm, perhaps the microorganisms in the biofilm would eat (and thus remove) the harmful organic material from the water.
Good idea! Indeed, even before biofilms were recognized and became the subject of intense research, engineers were taking advantage of natural biofilm environmental activity (without knowing about biofilms) in developing water-cleaning systems. Biofilms have been used successfully in water and wastewater treatment for well over a century. English engineers developed the first sand filter treatment methods for both water and wastewater treatment in the 1860s. In such filtration systems the filter medium (i.e., sand) presents surfaces for the microbes to attach to and to feed on the organic material in the water being treated. The result? The formation of a beneficial biofilm that eats the "bad" stuff in the water, effectively filtering it. Of course, we don't want the microorganisms in the biofilm to get into the filtered water, or for chunks of biofilm to detach from the colony and make it through the system. Ideally, the biofilm stays attached to the filtration system and can be cleaned when the system is flushed.
Interestingly, scientists and water treatment engineers have discovered that drinking water and wastewater that have been processed with a biofilm system in a treatment plant are more "biologically stable" than water filtered by other types of treatment systems. This just means that there is likely to be less microorganism contamination in water that has passed through a biofilm-based filter than there is in water that has passed through some alternative treatment system. This implies that biofilm treated water typically has lower disinfectant demand (e.g., use of chlorine) and disinfection by products (e.g., that unsavory taste and smell of chlorine) than water treated in other ways if the water prior to treatment is high in the kind of nutrients the biofilm craves (which in this case is organic carbon).
People are finicky. We want our drinking water to be crystal clear, have no odd odor, and to taste like pure water. Water that is safe to drink because of being treated with chlorine can still have an odd color, smell bad, and taste worse. So, drinking water utilities go to great lengths to provide us with the kind of drinking water we want (using ozone in the primary treatment phase is one approach that is used). In any such system, a biofilm treatment phase may well be one approach that will help yield the desired result.
Remediation of contaminated soil and groundwater
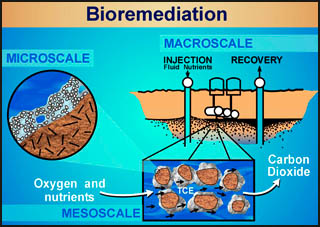
One of the less obvious beneficial applications of biofilms is in cleaning up oil and gasoline spills. That's right, certain bacteria will eat oil and gasoline. Remember that oil was produced over many years by decaying vegetation, so it is an organic compound. We wouldn't recommend that you suck up any spilled oil or gasoline, but the fact that some of the naturally occurring bacteria in soil love the stuff leads to a new idea: bioremediation. This is a term that refers to the engineering of a biofilm that can be introduced into the area of an oil or gasoline spill to help clean up the mess, and all with natural, non-harmful means.
Indeed, bioremediation using biofilms has emerged as a technology of choice for cleaning up groundwater and soil at many sites contaminated with hazardous wastes. Bioremediation results in
- the reduction of both contaminant concentration and mass for many subsurface contaminants (e.g., petroleum hydrocarbons and chlorinated organics) and/or
- a beneficial speciation change in the bacteria in the biofilm that allow them to tackle other contaminants, such as heavy metals (e.g., mercury)
In other words, bioremediation is a great idea! How to actually make it work requires an understanding of biofilm processes and engineering systems for introducing a biofilm into the contaminated ground and providing the necessary environment below the surface of the ground to encourage the biofilm to do its job (illustrated in the diagram above). For students interested in this topic, the study of biofilms and engineering (e.g., environmental engineering or chemical engineering) is where you want to be. Just keep on truckin', and you will get there.
Microbial leaching

Photo courtesy of Kennecott Utah Copper, Salt Lake City, Utah. This image shows a vast complex of leaching fields in which ore of low copper content is sprayed with water. Bacteria such as Thiobacillus, attached to the surface of the ore particles oxidize the insoluble copper compounds to soluble copper sulfate from which the pure copper can easily be recovered.
As you probably know, mining for precious metals of various kinds (gold, silver, copper and so forth) is a messy job. The desired metal is not generally found in nice, big, pure chunks. The largest gold nugget ever found was reputed to weigh about 70 Kilograms. But most gold, as with all other precious metals, is generally hard to see with the naked eye, mixed in the ground with dirt, rocks, and other ground debris—the ore from which the gold must be extracted (note that the ore in a good copper mine, for instance, will typically consist of less than 1% copper). The extraction process, when done with chemicals, is called "leaching." For years, the leaching of copper, for example, was done with acid which is not very good for the environment. In fact, most leaching technologies have resulted in toxic leftovers.
Well, guess what? Today approximately 10 to 20 percent of copper mined in the United States is extracted from low grade ore with the assistance of biofilms. And mining companies are making a considerable investment to extend this process to the extraction of other precious metals.
How is a biofilm engineered to accomplish this job? Again, one must find a bacteria with a particular appetite—one that would eat the ore, say, that encased copper particles, thus releasing the copper to be recovered. This idea has led to the most common biofilm supported leaching process, called "heap leaching." Low grade ore is placed in a "heap," and sprayed with a mildly acidified water solution that encourages the growth of a particular bacterium that oxidizes the ore, releasing water soluble cupric ion (copper) that can then be recovered from the water.


