A substantially updated version of the hypertextbook is available here. Please migrate to that version. This one will eventually disappear.
How is the skin healing process affected in chronic wounds?
An overview of the normal skin healing process
Following an injury to the skin, inflammation occurs, bringing platelets to form a blood clot, leukocytes to combat microbial invaders, and mesenchymal cells that develop into fibroblasts. Then the migratory phase begins, a scab is formed and epithelial cells migrate across the wound under the scab. Granulation tissue is formed as fibroblasts produce extracellular matrix and endothelial cells form blood vessels. The migratory phase is followed by the proliferative phase, characterized by extensive growth of epithelial cells, fibroblast deposition of collagen fibers in random patterns, and continued growth of blood vessels. Finally, the maturation phase occurs where collagen fibers become more organized, blood vessels are restored to normal, the scab is shed, and the epidermis is restored to normal thickness. The simple description of wound healing described above belies the fact that the process of wound healing is extremely complex, involving hundreds of growth factors, dozens of integrins, scores of enzymes, and over ten different cell types.
Diagram of normal skin healing
Absolute and relative barriers to healing
A number of factors can impede the healing process: low blood oxygen content, infection, lack of perfusion, sustained pressure, patient malnutrition, systemic disease such as diabetes and treatments such as immunosuppressants. Though any one of these factors can become an absolute barrier to healing, most often they are relative barriers to healing. For example, infection (evidenced by pain, erythema, heat, edema and tissue necrosis) can be an absolute barrier, but in most wounds it is a chronic process, waxing and waning—in other words, a relative barrier. Likewise, pressure can produce direct cell damage or impair perfusion. If pressure in the wound area exceeds capillary closing pressure, there is no blood flow, resulting in an absolute barrier to healing. But pressures tend to be intermittent, making them a relative barrier to healing. Typically all of these barriers occur as relative barriers to healing, but they appear in combination, compounding their effects.
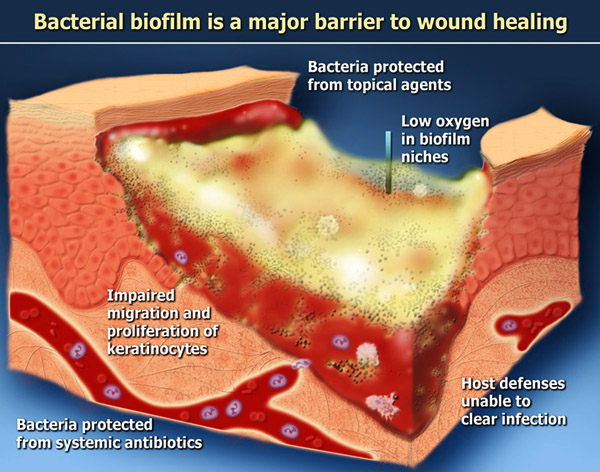
How the healing process is disrupted in chronic wounds
In the case of chronic wounds, the normal healing process is disrupted in numerous ways. Fibroblasts isolated from chronic wounds show impairment of synthesis, migration and proliferation. Endothelial cells from chronic wounds are deficient in production of enzymes and growth factors, and also are impaired in migration, proliferation, and the formation of new capillaries. Similarly, keratinocytes are impaired in their migration and proliferation as well as their ability to synthesize cytokines, provisional matrix and basement membrane.
When matrix metalloproteases are evaluated in chronic wounds, regardless of the etiology, and regardless of the age, gender, or any other demographic factor of the patient, they all show an identical biochemistry. Matrix metalloproteases are up regulated up to 30-fold. There are certain patterns of increases in matrix metalloproteases: matrix metalloprotease 8 (neutrophil drive) and matrix metalloproteases 2 and 9 (macrophage drive) seem to be the most upregulated. Of the four tissue inhibitors of metalloproteases (TIMP), tissue inhibitor metalloprotease 1 seems to have the most importance. This is ubiquitously downregulated in chronic wounds, producing a hallmark feature of chronic wounds—a very high ratio of matrix metalloprotease to MMP to TIMP. Other findings in the chronic wound environments seem to be secondary to the above phenomena. There is a significant decrease in growth factors and cytokines in general due to proteolytic degradation; there is a reduction in functional receptors on the somatic cells making up the wound bed resulting in their senescence; and there is a marked increase in proinflammatory cytokines such as interleukin 1, tumor necrosing factor alpha and gamma interferon.
The presence of proinflammatory cytokines is interesting and may be interpreted as a response to foreign agent. The innate immune system is designed to be vigilant and reactive to foreign insults, especially bacteria. Toll-like receptors (TLR) 2 and 4 are very sensitive to lipopolysaccharide (LPS) material produced by Gram-negative bacteria as well as teichoic acid, produced by Gram-positive bacteria. When LPS or teichoic acid stimulates the receptor, an exaggerated response yielding proinflammatory cytokines, interleukin 1, tumor necrosing factor alpha and gamma interferon results. These are chemotactic and reductive biochemicals which cause the migration and stimulation of the innate immune system, leading into mobilization of cellular and humoral immunity.
Host defenses relative to biofilm
Host defenses are robust, redundant and complex; this discussion will be limited to a few of the immune system components in the context of biofilm. The mammalian immune system is geared to provide surveillance against any foreign invader, but especially bacteria. The tool-like receptors are sensors looking for the fragments of gram-negative bacteria (LPS) or gram-positive bacteria (teichoic acid). Once these molecules are identified, a very potent immune system response is generated. Through many intracellular intermediaries, the dendritic cells (tissue macrophages) produce proinflammatory cytokines such as interleukin 1, tumor necrosing factor alpha, interleukin 8, interleukin 12, interleukin 6 and others. Collectively these are termed proinflammatory cytokines, which are dramatically elevated in all chronic wounds. The effect of these proinflammatory cytokines is to produce a swarming of the area with neutrophils and macrophages. If this defense system is in any way delayed, the individual bacteria have time to attach to the surface and enter the biofilm mode of growth.
Toll-like receptors, along with antibodies, are important early host defenses. Once biofilms form, antibodies no longer attach to the bacteria within the microcolonies. Experiments in cystic fibrosis using antibody stains show antibodies thickly crusted on the outside of biofilm, but not within the biofilm itself.
Studies on white blood cell activity against biofilms have demonstrated similar findings.
Laboratory experiments tend to suggest that antibodies, white blood cells and other immune components are ineffective against biofilms. However, clinical evidence shows that patients with biofilm-based infections can sometimes heal. For instance, children with chronic otitis media usually clear their ear infections with time. Many chronic wounds, even when inadequately treated, will go on to heal. Clearly there are host factors at work suppressing biofilm. However, patients who remain impaired, whether due to poor perfusion, repetitive trauma, poor nutrition, poor oxygenation or white cell dysfunction will need help in suppressing the biofilm and addressing other barriers that prevent wound healing.
