Harvesting and Dispersing of Cells from Biofilms (Alternative Method) - Instructions for Students
A substantially updated version of the hypertextbook is available here. Please migrate to that version. This one will eventually disappear.
Copyright © Alfred B. Cunningham, John E. Lennox, and Rockford J. Ross, Eds. 2001-2008
Harvesting and Dispersing of Cells from Biofilms (Alternative Method)
Supplies Needed
|
Quantity |
Description |
| 1 | wooden applicator stick |
| 4 | microcentrifuge tubes 1.5 ml |
| 1 | flask containing sterile distilled water or
phosphate buffered saline, for making dilution
tubes |
| 1 | mechanical pipetting device and sterile pipette or an automatic pipetter, pipette and pipette tip |
| 1 | biohazard bag for disposal of used tips |
| 1 | Vortex mixer |
| 1 | Branson or equivalent sonic cleaning water bath |
| 1 | forceps/hemostat |
| 1 | microscope slide, coupon or other object with biofilm attached |
Instructions:
Harvesting Cells
- Using a sterile pipette and mechanical pipetting device, dispense 0.9ml (900ml ) of distilled water or phosphate buffered saline (PBS) into sterile microcentrifuge tubes (1.5ml). These will be used as dilution blanks.
- Select a slide or coupon bearing an established biofilm. Hold the slide with a flame sterilized hemostat/forceps.
- Remove the cap from the tube containing the sterile wooden applicator sticks and gently shake the sticks so that one end extends a short distance from the tube. Being careful to touch only one stick, remove it from the tube.
- Using the applicator stick as a scraper, rub the surface of the coupon for approximately 15 seconds. Be sure to hold the stick perpendicular to the coupon surface so that the flat end of the stick is doing the scraping. Occasionally transfer the material removed to one of the 0.9 ml microcentrifuge tubes prepared previously. Swirl the applicator stick vigorously to remove the biofilm. Note: you may not be able to see any visible material on the stick or in the diluent as the stick is rinsed. Repeat this process at least 3-4 times (as indicated by your instructor) to ensure full coverage of the coupon surface or defined area being sampled.
- Using a sterile pipette and automatic pipetting device, rinse the scraped area once with 10 ml aliquots of sterile distilled water of PBS. This rinse water should be added to the 0.9 ml dilution blank. The total volume of rinse water should be 1 ml, (for example rinse 4 times with 10ml aliquots, then add 60 ml to bring the tube up to 1 ml).
- Dispose of the contaminated applicator stick, pipette, scraped slide in the biohazard bag provided.
Dispersing Cells: Sonicator Method
This technique uses a Fisher Scientific, a Branson Ultrasonic Corporation or similar sonic water bath cleaner delivering approximately 50-60 hz, to disrupt biofilms and disperse cells.
- Insert the microcentrifuge tubes (containing the biofilm harvested in the previous section) into a floating device, such as a thin piece of StyrofoamTM with holes for the tubes to be inserted. The floating device will hold the tubes upright while in the sonic water bath.
- Sonicate the tubes for 2 minutes. Turn off sonicator before removing tubes. Note: The time required for disruption of the biofilm varies with the material and the appropriate time for any particular specimen should be determined by the instructor.
- The cells should now be dispersed and ready for the next procedure.
- The sonicated microcentrifuge tubes should be vortex mixed before proceeding to dilution and plating to ensure that the dispersed cells are uniformly distributed.
Illustrations:
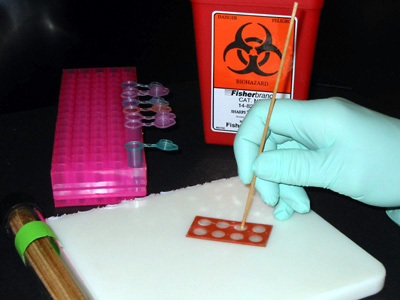
Figure 1. Scraping a coupon.
Educational Program Curricula and Teaching Resources
Supported in part by the Waksman Foundation for MicrobiologyDeveloped in collaboration with Dr. John Lennox, Penn State University-Altoona
©1999-2006 Center for Biofilm Engineering, http://www.erc.montana.edu