A substantially updated version of the hypertextbook is available here. Please migrate to that version. This one will eventually disappear.
Henrici’s Microbial Fishing Technique1
Introduction
“Some time ago an aquarium in my laboratory developed on its walls a growth of algae. Some microscope slides were placed in the aquarium in the expectation that the algae would also grow on these and that permanent preparations might be made showing the organisms in situ. On removing and staining these slides after a week's immersion, I was surprised to find, in addition to the algae, a thin and uniform coating of bacteria of various forms, some of unusual morphology”. … “It is quite evident that for the most part the water bacteria are not free floating organisms, but grow upon submerged surfaces; they are of the benthos rather than the plankton”. Arthur T. Henrici1.These comments written in 1933 indicate, that long before the term biofim was used2, Henrici understood the basics of biofilm formation. Elsewhere in that same article Henrici showed that he had an understanding of biofilms that sounds modern even by contemporary standards.
This understanding included the facts that 1) the bacterial cells are actually growing on the slide as confirmed by the presence of microcolonies, 2) they are firmly attached to the glass slide, 3) the cells are surrounded by a matrix (sheath of gum) which holds the cells to the slide, 4) specimens from aquaria and lakes alike produced these biofilms, 5) most bacteria are not in the water column but rather are surface attached.Using techniques very much like those described by Henrici it is possible to “fish” for bacteria, algae, protozoa and microinvertebrates by placing clean glass microscope slides in an aquatic environment such as aquaria, lakes, streams, showers, the tanks of toilet bowls, and any other site where there is a large or small body of water either standing or flowing.
Supplies Needed
|
Quantity |
Description |
| As Necessary | 18 gauge wire |
| 1 | wire cutters and pliers and shears |
| 1 | spine from a student report binder |
| As Necessary | rubber bands |
| As Necessary | microscope slides (or preprinted slides from Erie Scientific or other distributer) |
| As Necessary | transparent tape or Parafilm® M |
Instructions:
Henrici placed microscope slides in aquaria and lakes in order to harvest a variety of living, surface-attached bacteria. This same approach can be employed by students today.With this device (Figure 1), single microscope slides can be anchored in natural aquatic environments where biofilms might form.
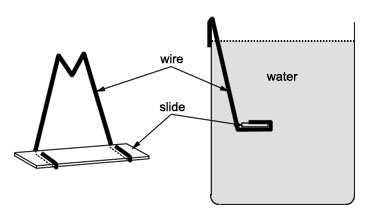
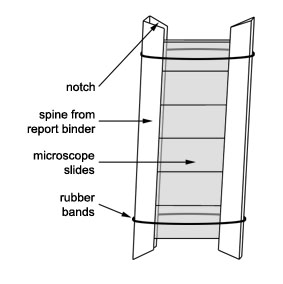
- The spine can be cut in half and about six clean microscope slides can be inserted into the two halves thus formed.
- The halves of the binder are held together by rubber bands.
- In this way six slides can be sited as a unit.
- Individual slides can be removed at intervals and examined for biofilm production.

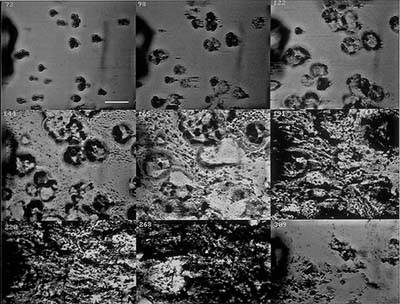
A multiple well slide can also be used in a microbial fishing exercise by masking all but one of the wells with transparent tape or Parafilm® M prior to placing the slide at its site. Then each day or at another selected interval, one additional well can be exposed. At the end of the sampling period each student has a sequence representing the growth of a biofilm over a period of time all on one slide.
REFERENCES
1Studies of Freshwater Bactria: I. A Direct
Microscopic Technique
Henrici, AT
Journal of Bacteriology 1933; 25:277-286
2How Bacteria Stick
Costerton JW, Geesey GG, Cheng K-J
Scientific American 1978; 238(1):86-95
3Flow induced vibrations, drag force, and
pressure drop in conduits covered with biofilm
Lewandowski Z, Stoodley P
1995. International IAWQ Conference Workshop on Biofilm
Structure, Growth, and Dynamics, 30 Aug. to 1 Sept.,
Noordwijkerhout, The Netherlands.
This material is based upon work supported by the National
Science Foundation under Grant No. 0618744, and in part by the
Waksman Foundation for Microbiology. Developed in collaboration
with Dr. John Lennox, Penn
State Altoona. Any opinions, findings, and conclusions or
recommendations expressed in this material are those of the
author(s) and do not necessarily reflect the views of the
National Science Foundation.
©2002-2008 Center for Biofilm Engineering, http://www.biofilm.montana.edu